Calculation of ion conductivity of oxide-based Lithium Ion Conductor LLZO via Self-Learning Hybrid Monte Carlo method#
self-learning hybrid Monte Carlo method [1][2]#
On the conventional method creating the neural network potentials (NNP), there are problems such as the arbitrariness in the method of creating random structures for training data and leaving it up to user that deciding how much training data should be increased.
The self-learning hybrid Monte Carlo (SLHMC) method creates NNPs with computational method shown below.
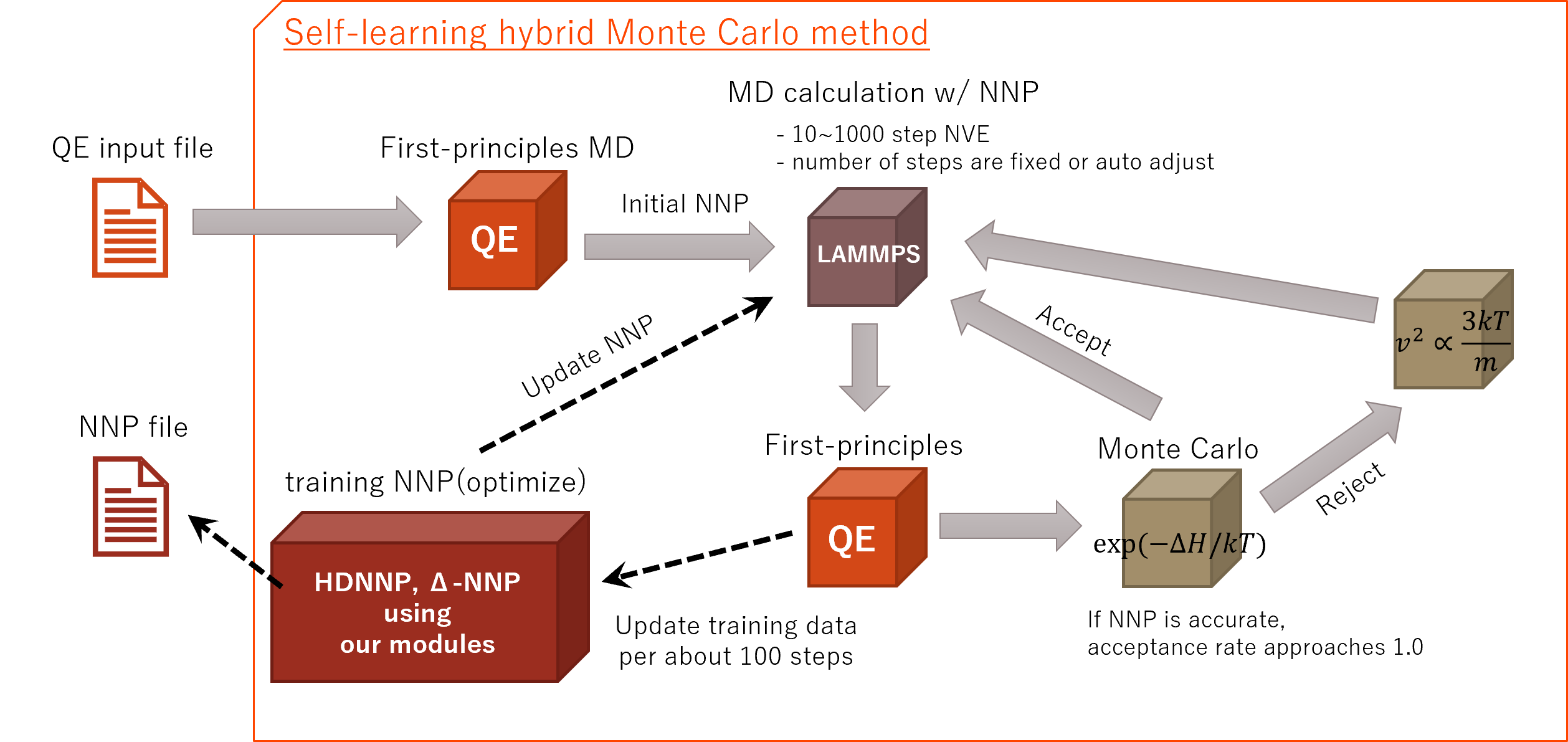
This method has advantages like that;
- It can always create same level NNPs independent of user's proficiency or creating procedure.
- There are few operations user should do because the calculation process is automated. Users should prepare only the input files of first-principles calculations.
- In addition to fewer operations, since the number of training data is tend to be reduced, the time which is required to creating NNPs can be greatly reduced. The process to take several weeks now will be half a day to several days.
- The physical quantities can be evaluated with accuracy of first-principles method using results of Monte Carlo calculation.
Advence/NeuralMD use Quantum ESPRESSO and LAMMPS (both are OSS) for first-principles and molecular dynamics (MD) calculation respectively. It use our proprietary modules for training NNPs. In addition, the ensemble for Monte Carlo method handles NVT and NPT, and there is the function to automatically adjusts the number of steps for MD calculation depending on acceptance rate.
All of these functions can be operated by Advance/NanoLabo (GUI).
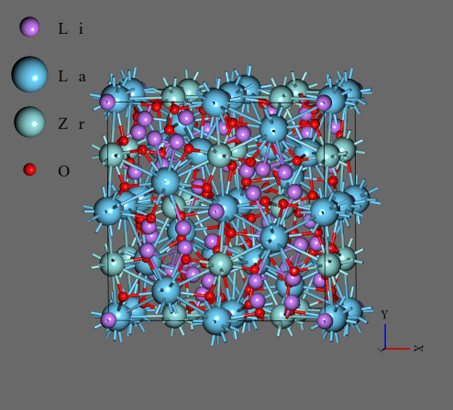
Oxide-based Lithium Ion Conductor: Li7La3Zr2O12#
Li7La3Zr2O12 (LLZO) is the oxide-based Li ion conductor which has ion conductivity about 2.5 x 10–1 S/cm at room temperature. It has a garnet-type crystal structure consist of 12 LaO8 dodecahedrons and 8 ZrO6 octahedrons, and 28 Li+ ions, in each unit cell.
LLZO has BCC (cubic) and BCT (tetragonal) structure. The ion conductivity of the BCT structure is greatly reduced to about 2.4 x 10–2 S/cm even the structural defferences of two structures are less than 2%. We created the NNP via SLHMC method using the LLZO in these two crystal structures as training data, and compared the ion conductivities.
Create Neural Network Potential#
We prepared the input data for Quantum ESPRESSO and execute SLHMC method.
- For initial structure, We used Li28La12Zr8O48 (96 atoms) system of BCC structure. As the crystal structure, the one obtained from Materials Project (mp-942733) was converted into primitive cell and BCC structure whose lattice constants are all about 11.2 Å.
- The MD calculation was done in NVT ensemble. To take the differences between BCC and BCT structures, NNP-MD in NPH ensemble was added per 200 steps. The temperature of MD calculation was settled at 800 K to obtain variations of structures.
- The density functional theory using ultrasoft pseudo potentials is used for SCF calculations, and GGA-PBE was used as a exchange-correlation functional. The cut-off energy and k-points were 36.0 Ry and Γ-point only respectively.
- 80 Chebyshev functions which has 5 Å cut-off radius was used as symmetric function in NNP. The structure of Neural Networks are 40 nodes x 2 layers, and the activation function was twisted tanh. In addition, the atomic charges were expressed with third-generation HDNNP, and Δ-NNP method was used together.
- The initial potential were created by calculating 200 steps of FPMD. NNP-MD were done in 10–80 steps (2.5–20.0 fs) per SCF calculations. The training data and NNP were updated every time when 100 training data was created. The training data include both of accepted and rejected, and the total number of them are 2500.
Using Intel Xeon Gold 5218 (2 CPU, 32 cores), the NNP was created at about 50 hours. When create the NNP of the similar Li ion conductor (LGPS) via conventional method, it took nearly a week. The CPU running time doesn't change significantly, but the total time was greatly decreased because the procedures are automated.
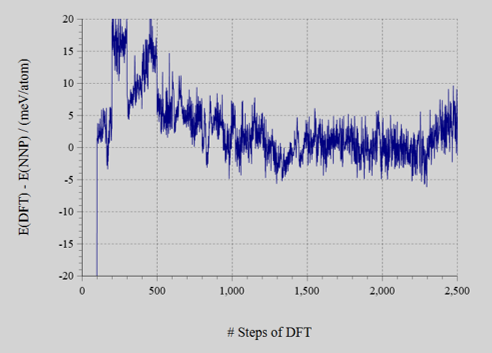
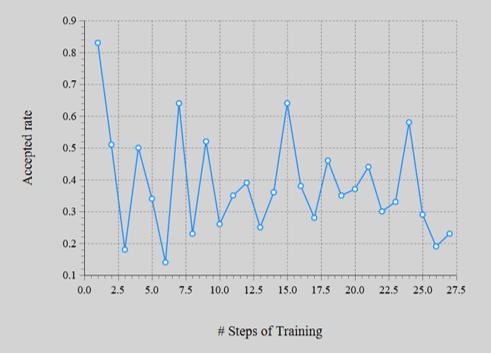
The energy error (left figure) is converged to MAE = 2.2 meV/atom. The acceptance rate of MC method (right figure) were in ranges 20–60%.
Calculate Ion Conductivity#
NNP-MD calculation was performed using the created NNP.
- The BCT structure unit cell of mp-942733 obtained Materials Project, and the BCC structure which changed mp-942733 into 12.95 Å lattice vectors are used. Both of them are 192 atoms system.
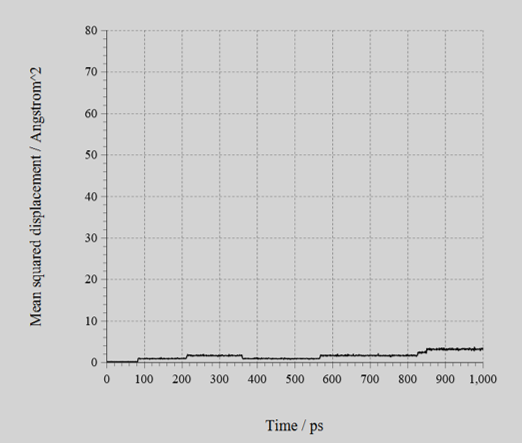
- MD calculations were done in isotropic NPT ensemble, at 600 K abd 1 ns, and calculate the ion conductivities from the diffusion coefficients .
The ion conductivity were calculated on followiong equation using the diffusion coefficient , the Li+ concentration , the elementary charge , the Boltzman's constant , the temperature .
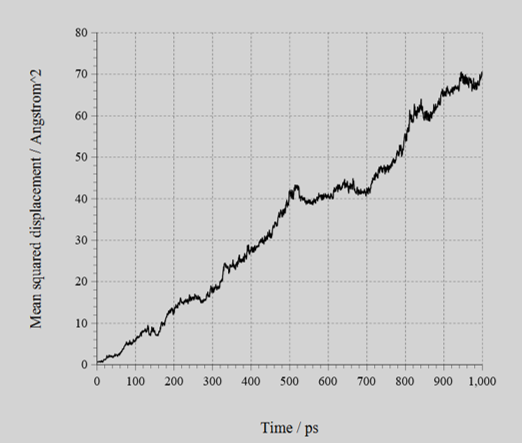
The MSD of Li+ (figure) and the calculated ion conductivities (table) is shown below. As shown, the difference of ion conductivity between BCC and BCT structures could be reproduced by the NNP created with SLHMC.
| σ in BCC structure / (S/cm) | |
|---|---|
| NNP-MD | 9.4 x 10–2 |
| Buckingham | 8.6 x 10–4 [3] |
| Experiment | 2.5 x 10–1 [4] |