Moleculer Dynamics Simulation of LiZnxMn2-xO4 using CHGNet#
CHGNet#
CHGNet is a material graph neural network force field that incorporates energy, force, stress, and magnetic moment into tensor quantities. A key difference from prior material graph implementations is the incorporation of magnetic moments in the tensor quantities, which enhances the ability to learn the occupied orbitals of electrons and describe the degrees of freedom of atoms and electrons.
The performance is comparable to state-of-the-art machine learning potentials, and the graph representation is highly versatile, covering the entire periodic table.
Deng, B. et al. CHGNet: pretrained universal neural network potential for charge-informed atomistic modeling. nature machine intelligence 5, 1031–1041 (2023). https://doi.org/10.1038/s42256-023-00716-3.
In this analysis, the following results of molecular dynamics calculations using M3GNet are also included for comparison.
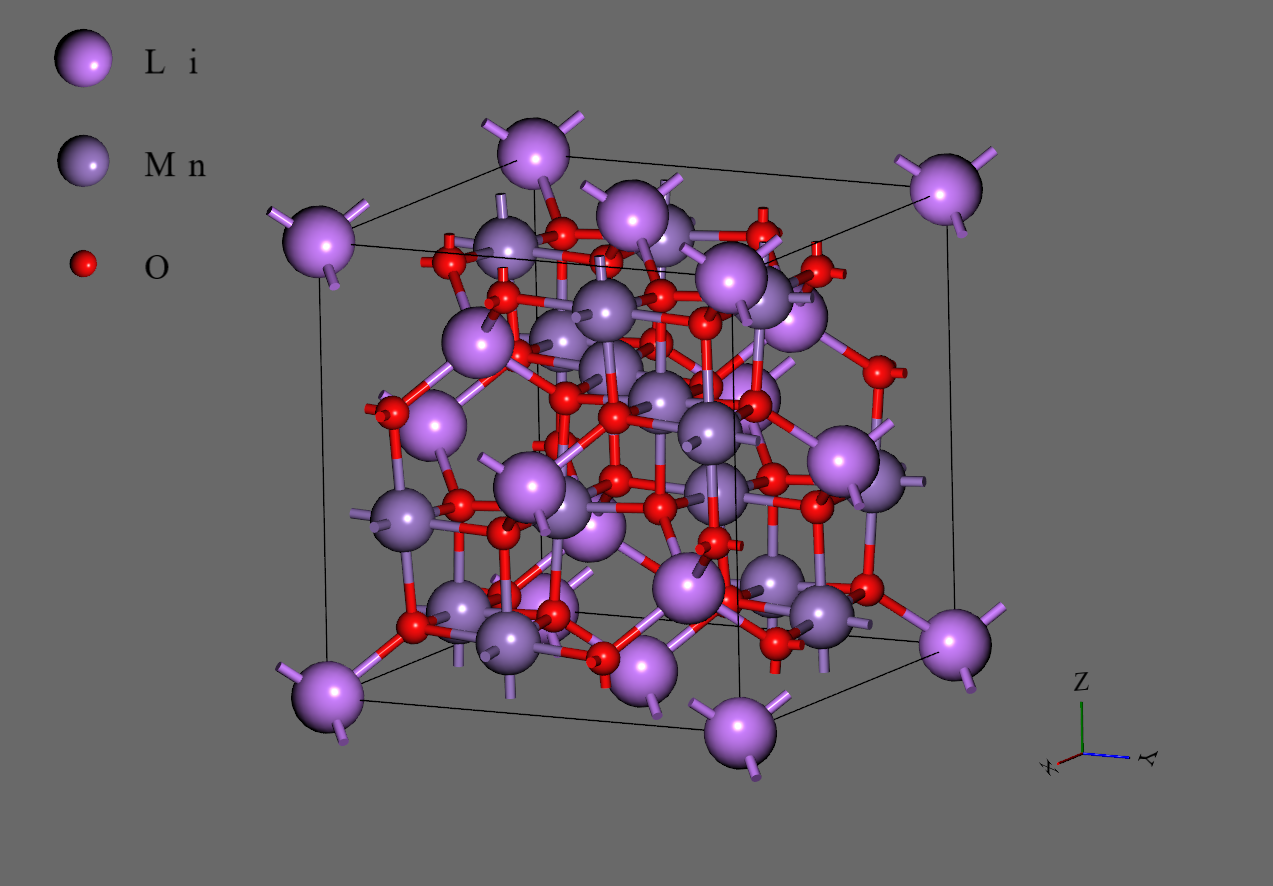
LiMn2O4#
Lithium ion manganese oxide LiMn2O4 is a spinel structure widely known as the cathode material of a lithium-ion battery.
Structural Phase Transition#
The LiMn2O4 structure changes from cubic at higher temperatures to orthorhombic, forming a 1×3×3 superlattice. This change is caused by the Jahn-Teller distortion of Mn3+O6 octahedra in LiMn2O4. In fact, both Mn3+ and Mn4+ ions exist locally and independently even in the high-temperature phase. It has been theorized that the regions where Mn3+O6 octahedra adjoin Mn4+O6 octahedra experience local distortion.
Hiromasa IKUTA, Yoshiharu UCHIMOTO, Masataka WAKIHARA, Crystal Structure Control of Lithium Manganese Spinel Oxides and Their Application to Lithium Secondary Battery, NIPPON KAGAKU KAISHI, 2002, 2002(3), pp. 271-280, https://doi.org/10.1246/nikkashi.2002.271
Substitution of Mn Atoms#
Increasing the ratio of Mn4+ by substituting some Mn with Co, Cr, Zn, etc., suppresses the Jahn-Teller distortion, which improves the stability of the cubic structure and lowers the transition temperature. It has also been reported that the lattice constants tend to decrease linearly as the substitution rate is increased.
Analysis Procedure#
CHGNet was applied to LiZnxMn2-xO4. Optimizing structures by changing the value of x, the following two were analyzed.
- Energies of cubic and orthorhombic structures.
- Lattice constants.
Structure Creation#
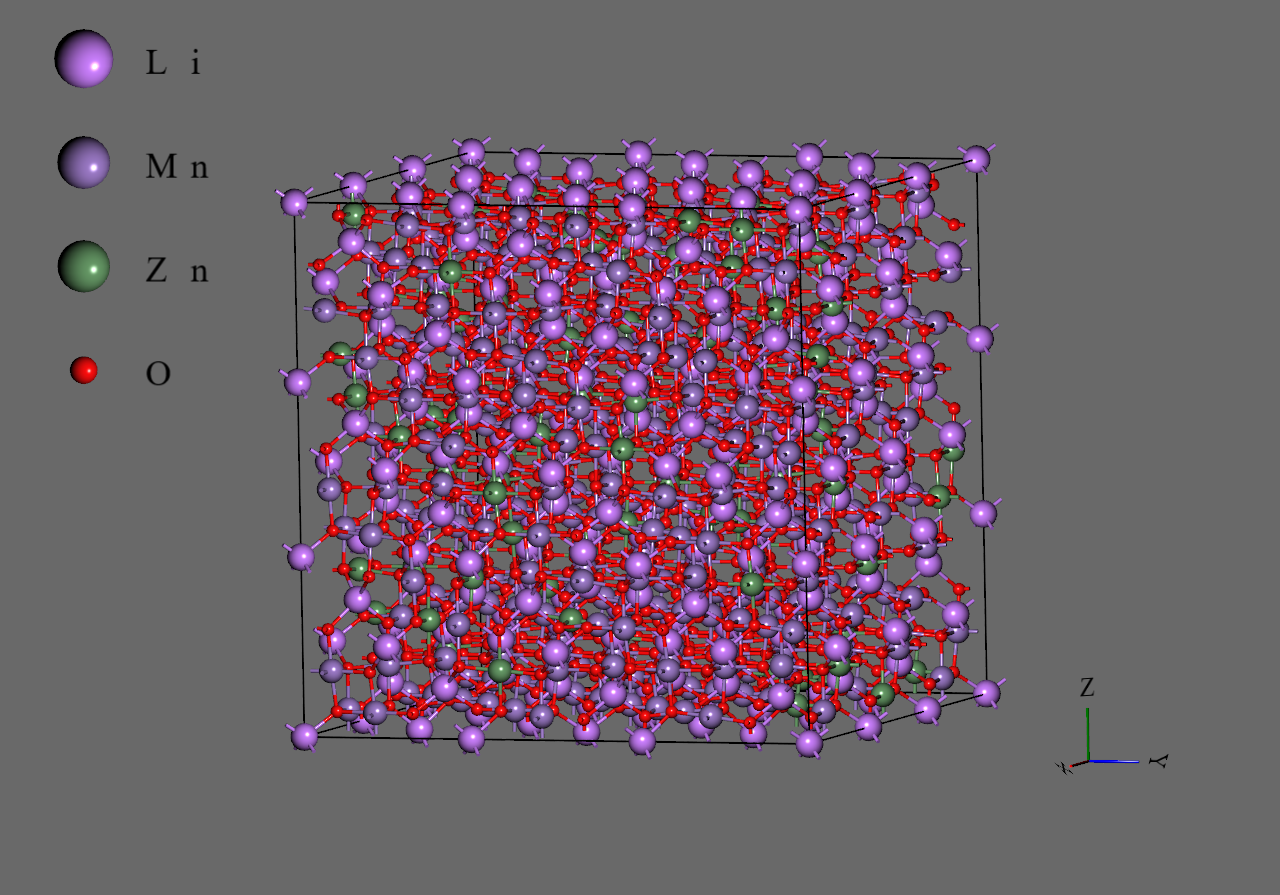
The basis structures were imported from Materials Project(https://materialsproject.org/).
- Cubic (mp-22584)
- Orthorhombic (mp-1199729)
Structures for analysis were created by randomly substituting Mn with Zn based on the above structures. The number of each structure is given in the table below. Since the orthorhombic structure forms a 1×3×3 supercell due to Jahn-Teller distortion, 3×1×1 orthorhombic structures were mapped to 3×3×3 cubic structures.
| x | 3×3×3 Cubic | 3×1×1 Orthorhombic |
|---|---|---|
| 0 | 1 | 1 |
| 0.1 | 5 | 5 |
| 0.2 | 5 | 5 |
| 0.4 | 5 | 5 |
| 0.8 | 5 | 5 |
Structural Optimization#
Structural optimization was performed while deforming the cell as follows.
| 3×3×3 Cubic | 3×1×1 Orthorhombic |
|---|---|
| Isotoropic | Anisotoropic |
Analysis Result#
Energies of Cubic and Orthorhombic Structures#
Calculating ΔEpot ( = Epotaniso - Epotiso ), the stability of cubic and orthorhombic structures can be evaluated relatively from the perspective of energy.
| x | ΔEpot (meV/atom) | ΔEpot (meV/atom)[M3GNet] |
|---|---|---|
| 0 | -31.85 | -11.06 |
| 0.1 | -7.693 | -5.777 |
| 0.2 | -3.557 | -3.251 |
| 0.4 | -1.075 | 0.3868 |
| 0.8 | 0.3498 | 0.2132 |
For x<0.4,, the orthorhombic structure was energetically stable. In addition, since the absolute value of the energy difference decreased as the substitution ratio increased, it was expected that the transition temperature decreased. These simulation results showed a similar tendency to the experimental results.
As described above, CHGNet could qualitatively reproduce the behavior of the phase transition.
Anisotropy of Orthorhombic Structures#
Anisotropy of orthorhombic structures was evaluated as the ratio of the magnitudes of the lattice vectors.
| x | Cella | Cellb | Cellc | Cella[M3GNet] | Cellb[M3GNet] | Cellc[M3GNet] |
|---|---|---|---|---|---|---|
| 0 | 1.0000 | 1.0092 | 1.0085 | 1.0000 | 1.0090 | 1.0094 |
| 0.1 | 1.0000 | 1.0073 | 1.0085 | 1.0000 | 1.0061 | 1.0078 |
| 0.2 | 1.0000 | 1.0037 | 1.0069 | 1.0016 | 1.0000 | 1.0074 |
| 0.4 | 1.0015 | 1.0000 | 1.0000 | 1.0000 | 1.0013 | 1.0018 |
| 0.8 | 1.0003 | 1.0008 | 1.0000 | 1.0018 | 1.0001 | 1.0000 |
It was found that a higher substitution ratio decreased the anisotropy of the orthorhombic structure. These results indicated a tendency where a higher substitution ratio of Mn stabilized the cubic structure, similar to the evaluation based on the energy differences.
Lattice Constants#
The lattice constants of cubic structures were evaluated.
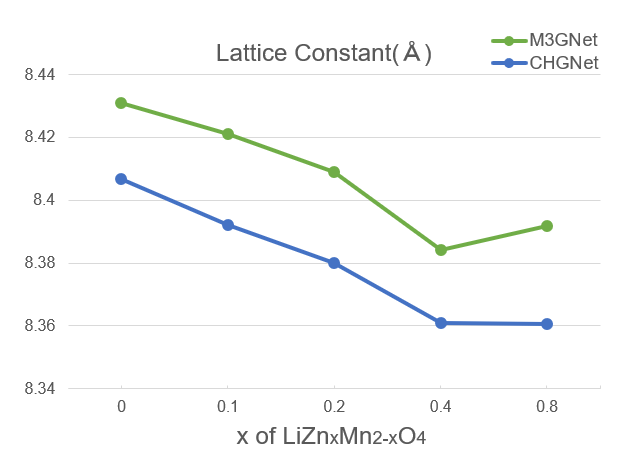
For x≤0.4,, it was observed that the lattice constant linearly decreased as the substitution ratio increased.
The above results showed that CHGNet could reproduce the effect of suppressing Jahn-Teller strain by substituting Mn with Zn and the accompanying tendency of the decrease in the lattice constant.